Describe the Causes and Consequences of Acid Precipitation
What does that mean. Precipitation collects acidic particles and gases and becomes acidic.
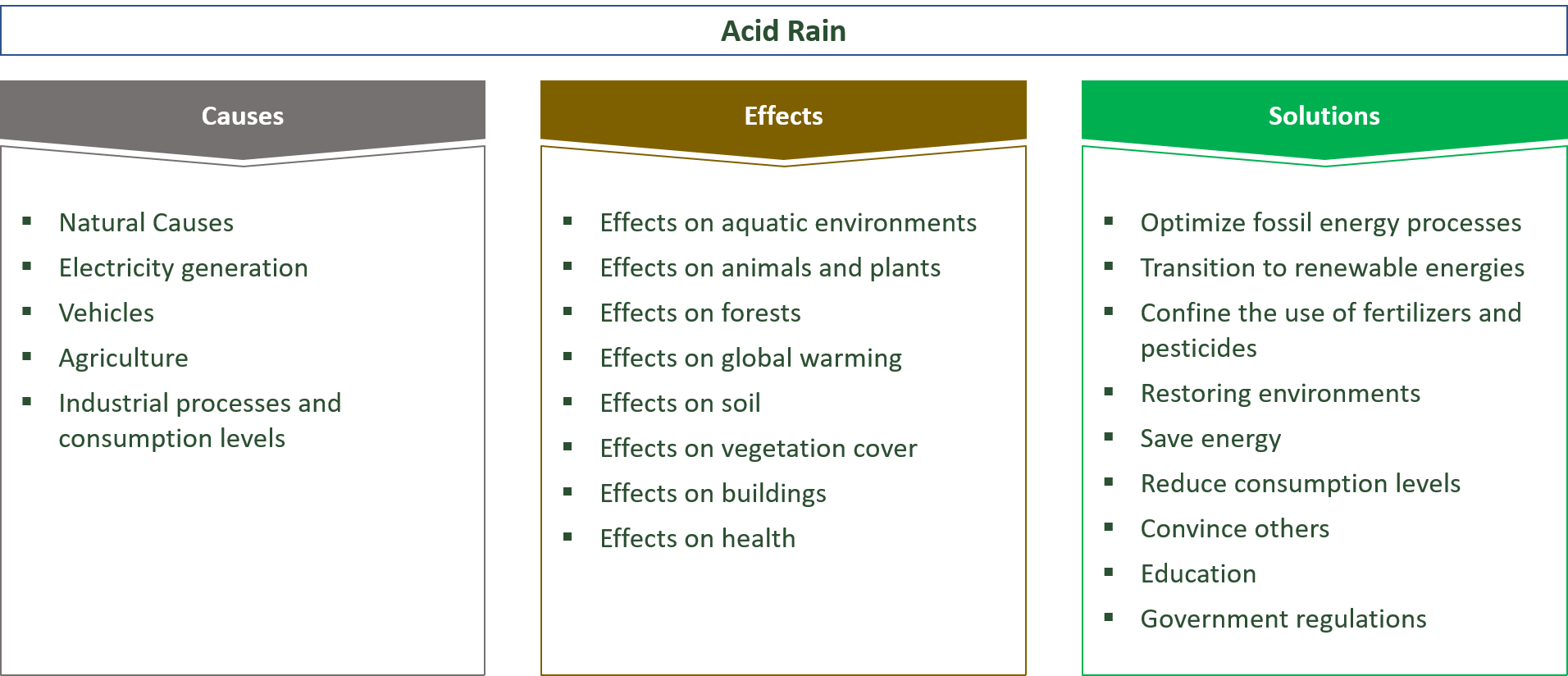
22 Causes Effects Solutions For Acid Rain E C
Acid rain is wet deposition.
. Causes of Acid Rain in Germany. Acidification of rain-water is identified as one of the most serious environmental problems of transboundary nature. It causes reduced rates of photosynthesis and growth increased sensitivity to drought and disease.
In general acid rain has an indirect effect on males. Acid rain is one of the consequences of air pollution. Has killed fish and damaged other forms of life in lakes and streams.
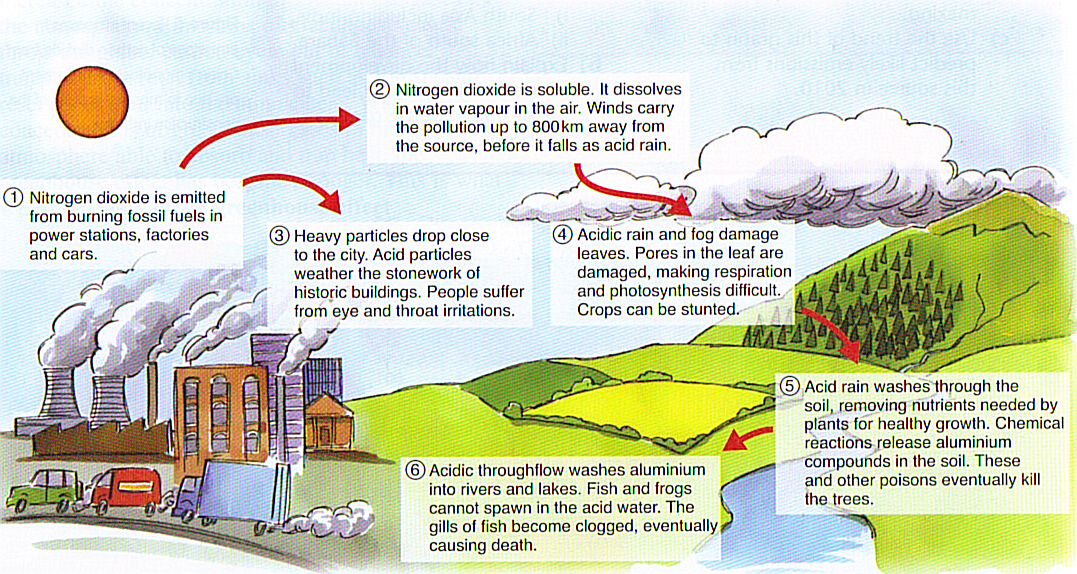
Because Germany is a top manufacturing country. Wet deposition is what we most commonly think of as acid rain. Gases produced from the burning of fuels react with the oxygen in the air and water vapour transforming into acids that fall onto the earths surface as rain.
Effects of Acid Rain on Plants and Trees. Acidic particles and gases can also deposit from the atmosphere in the absence of moisture as dry deposition. Acid rain also removes minerals and nutrients from the soil that trees need to grow.
Acid rain is one of the consequences of air pollution. Acid rain is the term commonly used by scientists to describe rain that is abnormally acidic. This process also occurs naturally through volcanic eruptions.
The precipitation is not necessarily. It leads the world in cars steel and chemical products. The major natural causal agent for acid rain is volcanic emissions.
This acidification of the earth and surface water has devastating effects on ecosystems and poses a serious danger to living beings. Sulfur oxides and nitrous oxides released by burning fossil fuels react with water in the air to form strong acids which fall to earth with rain or snow. Acid rain causes a cascade of effects that harm or kill individual fish reduce fish population numbers completely eliminate fish species from a waterbody and decrease biodiversity.
Dead or dying trees are a common sight in areas effected by acid rain. Dry deposition happens in both dry and wet weather so it could be called invisible acid rain No adequate way. Causes of Acid Rain.
As acid rain flows through soils in a watershed aluminum is released from soils into the lakes and streams located in that watershed. Since rain naturally has things dissolved in it it will always be slightly acidic. Acid rain is known to have many harmful effects on aquatic life plants animals.
In other words if acid rain does not cease and we consume such foods we can become very ill. The water collected from such rains is usually acidic rather than neutral. The primary cause of acid rain is the emission of nitrogen oxide and sulfur dioxide into the atmosphere.
Acid rain is caused by emissions of compounds of ammonium carbon nitrogen and sulphur which react with the water molecules in the atmosphere to produce acids. Necrosis dieback chlorosis defoliation. Cars and buses also produce harmful gases.
Acid rain can be defined as rain or any other kind of precipitation that is unusually acidic which means that it has higher levels of hydrogen and thus a lower pH-score. Building structures made of marble and limestone are mostly affected by acid rain as the acid eats the calcium compounds in the structures. The sulfuric and nitric acids formed in the atmosphere fall to the ground mixed with rain snow fog or hail.
It is caused by emissions of nitrogen oxide and sulfur dioxide which react with water molecules in the atmosphere and produce acid rain. As a result it has led to weathering of buildings corrosion of metals and peeling of paints on surfaces. Acid rain leaches aluminum from the soil.
The various gases like sulphur dioxide and nitrogen dioxide react with water vapours in presence of sunlight and form sulphuric acid and nitric acid mist. Dry deposition occurs when small acid particles and gases fall onto the earths surface. Acid rain has the following reaction with the marble calcium carbonate.
Describe the causes and consequences of acid precipitation and ocean acidification. Well plain distilled water like that used in laboratories is neutral not acidic or basic. Main source of acid rain is pollution from factories burning fossil fuels like natural gas coal and oil.
These substances can rise very high into the atmosphere where they mix and react with water oxygen and other chemicals to form more acidic pollutants known as acid rain. Wet deposition causes erosion that affects ecosystems. Sources of Acid Rain Acid rain is caused by a chemical reaction that begins when compounds like sulfur dioxide and nitrogen oxides are released into the air.
Acid rain or acid deposition is a broad term that includes any form of precipitation that contains acidic components such as sulfuric acid or nitric acid. Acid rain is primarily caused by the chemicals sulphur dioxide and nitrogen oxide. Shows strong acidity ie pH 45 or lower and gives rise to the term acid rain In dry deposition the acid remains in the form of aerosol particles or gas and is carried away by the wind before finally settling on the surfaces of water leaves soil or buildings.
It occurs when emissions from factories cars or heating boilers contact with the water in the atmosphere. Both natural and man-made sources are known to play a role in the formation of acid rain. Acid rain has corrosive effects because it eats into metals and stone.
The acidic particles and gases may deposit to surfaces water. It can also have an impact on people because the acid is found in fruits plants and animals. Acid rain is a term that refers to rain or precipitation containing elevated hydrogen ion concentrations.
These emissions contain nitrogen oxides sulfur dioxide and sulfur trioxide which when mixed with water become sulfurous acid nitric acid and sulfuric acid. CaCO3 H2SO4 - CaSO4 H2O CO2. Due to the interaction of these acids with other constituents of the atmosphere protons are released.
Acid rain damages plants direct effect on foliage and growing point. That aluminum may be harmful to plants as well as animals. Pollution is when gases smoke and chemicals are introduced into the environment in large doses that makes it harmful for humans animals and plants.
But it is mainly caused by the combustion of fossil fuels which results in emissions of sulfur dioxide SO 2 and nitrogen oxides NO x. Sulfur dioxide and nitrogen oxides dissolve very. These particles will have a pH level below 56.
Acid rain is mainly a mixture of sulphuric and nitric acids depending upon the relative quantities of oxides of sulphur and nitrogen emissions. There are two types of deposition processes.

Causes And Effects Ozone Depletion In Our Stratosphere The Greenhouse Effect And Uvc Rays Swiss Chemistry Vormatur Ozone Layer Ozone Depletion Ozone Layer Healing

Ess Topic 6 4 Acid Deposition Amazing World Of Science With Mr Green

No comments for "Describe the Causes and Consequences of Acid Precipitation"
Post a Comment